|

History
Department of immunotherapy and Leishmania vaccine research (former Molecular Immunology and Vaccine Research lab), was founded in 1998 by WHO-TDR support with a general objective of research on Leishmania immunology and vaccine. Most honorably, during past years, this department has recieved remarkable financial supports from WHO and TDR which has promoted both national and international collaborations on fundamental and applied Leishmania research. Since founding, this department has provided a stimulating environment apt for training graduate and doctoral students interested in Leishmania research who are fully appreciated as experts in their fields.
|
Committed to missions, over 70 original articles and
10 reviews are published in high ranked journals
and 7 different genes of Leishmania are registered
in Gene bank. By introduction of “Leishmania
Genome Manipulation” techniques, 5 national
patents are registered around transgenic
Leishmania which are among the very precious
achievements of the unit revolutionizing both
preventive and therapeutic research pertinent
to missions.Above all is a promising outcome
achieved by labor intensive hard work of
researchers in this unit which is a prototype
of a genetic vaccine against visceral
leishmaniasis that has passed experimental
evaluations in dogs
|
.JPG) |
In addition to basic research on Leishmaniasis and in commitment to general visions of Pasteur Institute of Iran, the unit has also started joint projects with clinical care units and hospitals in Capital or in endemic areas of Iran to help clinicians to speed up precise diagnosis of infection and appropriate treatment. Honorably, whole expertise of researchers and technicians working in this department is now open to similar fields both inside and outside Pasteur Institute of Iran regarding other intracellular microorganisms.
Objectives
Immunotherapy and Leishmania vaccine research department includes 3 different laboratories,.
- Molecular immunology and vaccine research lab,
- Recombinant microorganisms,
- Synthetic compunds and drug screening.
And is totally focused on its main objectives
- Vaccine development by screening novel antigens of both parasite and vector,
- ntroducing antigens for diagnostic purposes for both CL and VL for local applications,
- Development of live attenuated or non-pathogenic live-vectored and reverse based genomic vaccines,
- Transgenesis and development of transgenic parasites,
- Evaluating efficiencies of new or established drugs by transgenic parasites both in vitro and in vivo,
- Research on effective adjuvants and immunomodulators for immunotherapy,
- Research on pathogenic factors and pathogenesis,
- Research on novel anti-microbial peptides both in vitro and in vivo.
Molecular immunology and vaccine research lab
Despite the relative success of drug therapy for leishmaniasis, increasing resistance against therapeutic agents, urges development of an effective preventive vaccine. This lab is mainly engaged with vaccine design and evaluation against both CL and VL and also with validation of novel therapeutic reagents for effective treatment. To achieve the goals, different antigens, adjuvants, delivery systems and prokaryotic/eukaryotic expression systems has been under intensive investigation harnessing advanced methodologies and with full interactive national and international collaborations. This lab has also been involved in research on viral or bacterial infections besides leishmaniasis relying on expertise of committed researchers and experienced technicians.
Major goals:
- Screening novel vaccine candidates both by conventional and reverse approaches and experimental validations in corresponding animal models (mice, hamster and dog),
- Live attenuated parasite development either as vaccine or for immunotherapy,
- Genetic manipulation of non-pathogenic Leishmania for live-vectored vaccine concept promotion,
- Polytope vaccine research,
- Evaluating novel delivery systems effective for Leishmania vaccine,
- Evaluating different adjuvants and immunomodulators both in vitro and in vivo.
Recent publications
- Vaccination with recombinant L7/L12-truncated Omp31 protein induces protection against Brucella infection in BALB/c mice, M Golshani, S Rafati, A Dashti, E Gholami, S D Siadat, M Oloomi, A Jafari, S Bouzari, 2015, Molecular immunology, 65 (2), 287-292.
- N. Seyed, T Taheri, Ch Vauchy, M Dosset, Y Godet, A Eslamifar, I Sharifi, O Adotevi, C Borg, PS Rohrlich, S Rafati. Immunogenicity Evaluation of a Rationally Designed Polytope Construct Encoding HLA-A*0201 Restricted Epitopes Derived From Leishmania major Related Proteins in HLA-A2/DR1 Transgenic Mice: Steps Toward Polytope Vaccine. PLOS One, 2014, 9(10), e108848.
- Enhanced protective efficacy of nonpathogenic recombinant Leishmania tarentolae expressing cysteine proteinases combined with a sand fly salivary antigen, F Zahedifard, E Gholami, T Taheri, Y Taslimi, F Doustdari, N Seyed, F Torkashvand, C Meneses, B Papadopoulou, Sh Kamhawi, J G Valenzuela, S Rafati, 2014, PLoS neglected tropical diseases, 8(3): e2751.
- Cationic solid–lipid nanoparticles are as efficient as electroporation in DNA vaccination against visceral leishmaniasis in mice, N Saljoughian, F Zahedifard, D Doroud, F Doustdari, M Vasei, B Papadopoulou, S Rafati, 2013, Journal Parasite immunology, 35(12): 397-408.
- Development of novel prime-boost strategies based on a tri-gene fusion recombinant L. tarentolae vaccine against experimental murine visceral Leishmaniasis, N Saljoughian, T Taheri, F Zahedifard, Y Taslimi, F Doustdari, A Bolhassani, D Doroud, H Azizi, K Heidari, M Vasei, N Namvar Asl, B Papadopoulou, S Rafati, 2013, PLoS neglected tropical diseases, 7(4), e2174.
- In silico analysis of six known Leishmania major antigens and in vitro evaluation of specific epitopes eliciting HLA-A2 restricted CD8 T cell response, N Seyed, F Zahedifard, Sh Safaiyan, E Gholami, F Doustdari, K Azadmanesh, M Mirzaei, N S Eslami, A Khadem, I Sharifi, S Rafati, 2011, PLoS neglected tropical diseases, 5 (9): e1295.
Transgenic microorganism's lab
One of the most important achievements in our lab was “Leishmania Genome Manipulation” methodology in 2008 which opened up new concepts both in vaccine design and drug validations by introducing transgenic Leishmania species. This lab has successfully registered 5 national patents all refering to transgenic parasites labor intensively developed by advanced knock-in techniques to intrinsically harbor reporter genes such as EGFP and Luciferase.
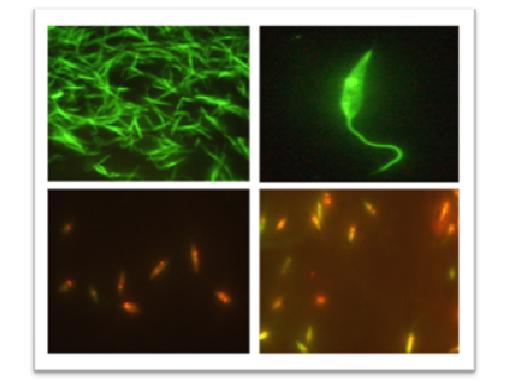
Recent publications
- Generation of stable L. majorEGFP-LUC and simultaneous comparison between EGFP and luciferase sensitivity, T Taheri, H Saberi Nik, N Seyed, F Doustdari, M H Etemadzadeh, F Torkashvand, S Rafati, 2015,Experimental parasitology,150: 44-55.
- Live vaccination tactics: possible approaches for controlling visceral leishmaniasis, N Saljoughian, T Taheri, S Rafati, 2014, Frontiers in immunology, 5.
- Fluorescent Leishmania species: development of stable GFP expression and its application for in vitro and in vivo studies, A Bolhassani, T Taheri, Y Taslimi, S Zamanilui, F Zahedifard, N Seyed, F Torkashvand, B Vaziri, S Rafati, 2011, Experimental parasitology, 127 (3): 637-645.
- Leishmania major: disruption of signal peptidase type I and its consequences on survival, growth and infectivity, T Taheri, A Salmanian, E Gholami, F Doustdari, F Zahedifard, S Rafati, 2010, Experimental parasitology,126 (2): 135-145.
Synthetic compounds and drug screening
This lab is mainly focused on novel treatment approaches based on novel therapeutics such as anti-microbial peptides, synthetic compounds and nanoparticles. For this high throughput methodologies are applied to evaluate anti-parasitic effects of therapeutic candidates in vitro, in vivo and ex vivo. Innovative approaches are ongoing in this lab based on nanoparticle delivered therapeutics and also immunization combined with novel therapeutics.

Major goals:
- Study of anti-microbial peptides both in vitro and in vivo,
- Study of novel synthetic compounds which restric amastigote proliferation using cell lines with both human and mouse genetic backgrounds,
- Labling anti-leishmania therapeutics for in-situ imaging promotion,
- Study of nanoparticle based therapeutics both in vitro and in vivo.
Recent publications:
- Human neutrophil peptide-1 (HNP-1): A new anti-leishmanial drug candidate, S Dabirian, Y Taslimi, F Zahedifard, E Gholami, F Doustdari, M Motamedirad, Sh Khatami, K Azadmanesh, S Nylen, S Rafati, 2013, PLoS neglected tropical diseases, 7 (10): e2491.
- Leishmaniasis: focus on the design of nanoparticulate vaccine delivery systems, D Doroud, S Rafati, Expert Review Vaccine 2012, 11(1): 69-86
- Cationic solid lipid nanoparticles loaded by cysteine proteinase genes as a novel anti-leishmaniasis DNA vaccine delivery system: characterization and in vitro evaluations. D Doroud, A Vatanara, F Zahedifard, E Gholami, R Vahabpour, A Rouholamini Najafabadi, S Rafati, 2010, Journal of Pharmacy and Pharmaceutical Sciences, 13(3): 320-35.
Major goals:
· Engineering precise constructs for genetic manipulation,
· Engineering transgenic parasites of different species by knock-in and knock-out recombination tecnology,
· Engineering recombinant non-pathogenic parasites expressing candidate available antigens,
· Stabilizing recombinant or transgenic parasites by lyophilization.
|